The ability to selectively activate specific cell types with artificial stimulation is highly desirable for many neural implants in that enhances control over neural signaling, i.e., elements of natural signaling can be more closely approximated with the prosthesis thus offering the potential of improving treatment efficacy. Achieving such control has proven challenging with conventional stimulation strategies however as most types of neurons in a targeted region have similar activation thresholds. In contrast to responses to single pulses, sensitivity differences have been observed across types in response to high frequency (> 1 kHz) stimulation (HFS). For example, peak firing rates occur at significantly different amplitudes for ON vs. OFF ganglion cells of the retina. HFS is also used in a host of other applications but surprisingly, many of the factors that govern responses remain incompletely understood. We are studying the responses to HFS with the goal of understanding the fundamental principles governing responses as well as the factors that shape sensitivity differences between types.
Background
High frequency (>1 kHz) electric stimulation (HFS) when delivered to axons blocks the transmission of spiking. While the suppression is thought to arise from HFS-induced depolarization block, there are competing theories, and the underlying mechanisms are not completely understood. In the retina, ON and OFF ganglion cells (RGCs) have different sensitivity to HFS, a highly attractive feature as it offers the potential that the out-of-phase signaling that occurs naturally between the two pathways can be replicated artificially. Here too, the factors mediating the sensitivity differences are not well understood and our goal is to better understand the biophysical mechanisms that govern sensitivity to HFS as a basis for rationally developing more effective stimulation strategies.
What We Do
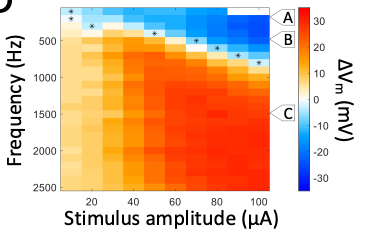
It has proven challenging to directly measure the neurophysiological response to HFS but the high impedances associated with patch clamp recordings enable responses to be recorded without saturation of the amplifier. Subsequent signal processing enables both the membrane voltage Vm (whole cell patch) and the spiking response (cell attached patch) to be completely captured. We are comparing Vm and spiking responses to a wide range of HFS parameters to determine the relationship between individual parameters and neuronal responses. In addition, we can correlate the relationship between Vm and spiking to directly assess the relationship between the two, i.e., whether depolarization and/or hyperpolarization mediate suppression. Use of the ex vivo retina is highly attractive because it enables measurements to be confined to specific, easily identified cell types and thus there is no confound between (potential) response differences between types. Perhaps more importantly, measurements across multiple cell types can be compares to determine the factors that mediate the previously observed sensitivity differences between types. Computational modeling is used to gain insight into the specific biophysical factors that govern sensitivity within and across types. Single- and multi-compartment models can be used to elucidate the relative importance of neuronal morphology vs. Hodgkin-Huxley kinetics. Immunochemical staining is used to evaluate model-based hypotheses, e.g., if specific types of voltage-gated ion channels mediate a given response, and the responses are different between types, we can assess whether the expression levels are different across types.
Selected Publication
Twyford, P, Fried, SI, (2015), The retinal response to sinusoidal electric stimulation. IEEE-Trans. Neural Syst. Rehabil. Eng. 2015 Apr 2. PMID: 25850091.
Raghuram, V, Werginz, P, Fried, SI (2019), Somatodendritic and AIS scaling in retinal ganglion cells helps to regulate spike properties and maintain response consistency, Front. Cell. Neurosci, https://doi.org/10.3389/fncel.2019.00436. PMID: 31611777